Finally came across a critical paper on mitochondrial health.
Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis (10/2023)
Introduction
Mitochondria serve as power plants that generate adenosine 5’-triphosphate (ATP) through oxidative phosphorylation (OXPHOS) for the cell. They also contribute to the regulation of calcium homeostasis, intracellular signaling transduction, cellular proteostasis, heme and lipid biosynthesis, reactive oxygen species (ROS) production, and programmed cell death [1,2,3,4,5,6]. To fulfill such diverse and critical roles in the cell, mitochondria undergo constant fission and fusion cycles to maintain their shape, network, and inheritance. This constant turnover helps maintain their fitness and normal cellular functions. Dysregulation of these critical processes has been causally linked to a myriad of diseases, including metabolic disorders, neurodegenerative diseases, heart and vascular diseases, inflammatory diseases, hematological diseases, and cancers [7,8,9,10,11,12].
Mitochondria are not generated de novo in eukaryotic cells. The preexisting mitochondria are distributed between the two daughter cells following cell division [13]. Mitochondria have circular DNA (mitochondrial DNA, mtDNA), and mitochondrial biogenesis (mitobiogenesis) involves the replication, transcription, and translation of mtDNA-encoded genes, the interorganelle transport of phospholipids, and the import of nuclear-encoded proteins into mitochondria through the protein translocation machinery of the outer and inner membranes [14]. Mitobiogenesis is a balanced process that also occurs in parallel to the process of removing mitochondria, ensuring that an optimal number of mitochondria persist within the cell. During evolution, cells have gained several strategies to monitor and remove damaged or superfluous mitochondria. One of the major mechanisms of removal is mitophagy, a selective form of autophagy that promotes mitochondrial degradation via the mitolysosomal pathway. In the past decade, both ubiquitin- and receptor-mediated mitophagy pathways have been described [15]. It is less clear how the two opposing processes of mitobiogenesis and mitophagy are coordinated at the molecular level and how they maintain a healthy population of mitochondria (Fig. 1). In this review, we discuss recent advances toward elucidating the molecular mechanisms underlying the coordination of mitobiogenesis and mitophagy. We review the molecular regulation of both mitophagy and mitobiogenesis processes and give special attention to the crosstalk between them that fine tunes the balance of mitochondrial mass and quality.
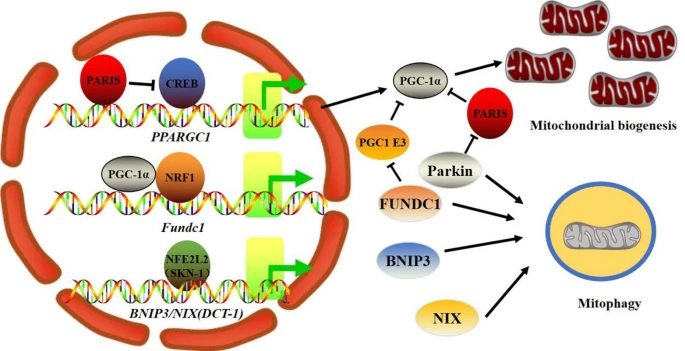
Coordination of mitochondrial biogenesis and mitophagy. PGC-1α is the primary regulator of mitobiogenesis, and in an NRF1-dependent manner, PGC-1α also regulates the expression of the mitophagy receptor Fundc1. The NFE2L2 homolog SKN-1 regulates the expression of the BNIP3/NIX homolog DCT-1 in C. elegans. In addition, PGC-1α activity can be decreased by mitophagy defects by either controlling its stability (Fundc1 deficiency) or suppressing its expression in a Parkin-PARIS-CREB pathway-dependent manner.
Concluding remarks
It is generally accepted that maintaining cellular and tissue homeostasis requires adequate mitochondrial number and function and that their dysregulation is intimately linked to the onset of many diseases. The regulation of mitochondrial mass and function is governed by two conserved processes, mitobiogenesis and mitophagy. If either one or both processes are disrupted, it results in an accumulation of dysfunctional mitochondria, oxidative stress, cellular aging, and ultimately cell death. Although initially considered two distinct processes, mitophagy and mitobiogenesis are now known to work in concert to maintain the health of mitochondria. More studies are warranted to investigate how cells maintain ideal quantities of mitochondria in response to multiple environmental and developmental signals. It will be interesting to see whether the factors involved in mitobiogenesis also affect the expression of other mitophagy-related proteins, in addition to the previously identified FUNDC1 and BNIP3. Future work will help to determine the mechanisms governing the feedback control of mitophagy dysfunction that leads to a deficiency in mitobiogenesis. As mitochondrial dysfunction is a key characteristic of a plethora of diseases, attempts to elucidate the crosstalk between mitobiogenesis and mitophagy will advance our understanding of the etiology and the development of novel therapeutic approaches for a variety of diseases.
https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-023-00975-7