adssx
#550
When you post in the bottom right corner of the picture you can click on “50%” to reduce its size.
3 Likes
adssx
#551
Optimizing timing and dose of exogenous melatonin administration in neuropsychiatric pediatric populations: a meta-analysis on sleep outcomes 2025
Our results suggest a dose and time of administration that may enhance melatonin’s sleep promoting effects (2-4 mg, 3 hours before bedtime) and, if replicated by large clinical trials, could guide clinical practice in managing sleep disturbances in children experiencing neuropsychiatric conditions.
Effect of acute administration of melatonin immediately after physical exercise on the amino acid profile of rat’s skeletal muscle and liver
Melatonin modulated the post-exercise amino acid profile in skeletal muscle, enhancing the levels of key metabolites involved in recovery and metabolic regulation, with no effects observed in liver tissue. These findings suggest a muscle-specific role for melatonin in supporting metabolic recovery after exercising.
2 Likes
adssx
#552
1 Like
My point about copper is that it is an essential mineral, but not in large quantities. I have been supplementing copper which has helped, but my copper levels continue to bump along the bottom of the normal range. That is because I drink from time to time. (as in now and whilst at the BSRA conference). Incidentally I don’t have an issue with the BSRA conference having alcohol as part of the conference, but interestingly it was hard to avoid potatoes and rice.
1 Like
adssx
#554
KiwiGuy
#555
I started mega dosing melatonin a few weeks ago. Estimate I am taking 1gm - 1.2gm per day (self filled caps, haven’t weighed them). Split in four equal doses - 1 hour before bed, At bedtime, 2x doses during nighttime if I wake. Not much to report. Seem to be tolerating it well. Sleeping better and more vivid dreams, not drowsy the following day or anything like that.
1 Like
KiwiGuy
#556
Something I am considering, to flatten/prolong a dose effect is to split it into 1x normal gel cap (quickly absorbed in stomach) and a second enteric coated cap (absorbed later in intestine). Wondering what people think of that idea? I am estimating each cap is somewhere around ~250mg - 300mg.
2 Likes
I fill my own caps as well because I use melatonin in such large quantities. Putting some in an enteric-coated cap might be a good idea.
Some day I would like to track the pharmacodynamics of supra physiological melatonin
LukeMV
#559
Great thread on X on why physiological dosing (some would call it microdosing) is the way to go. I’m a 0.3mg guy too.
https://x.com/keithsakata/status/1971203645300453515?s=46
Alpha
#560
The problem being finding enteric-coated capsules.
Anyone come up with a source?
Amazon has empty enteric-coated capsules in a variety of sizes.
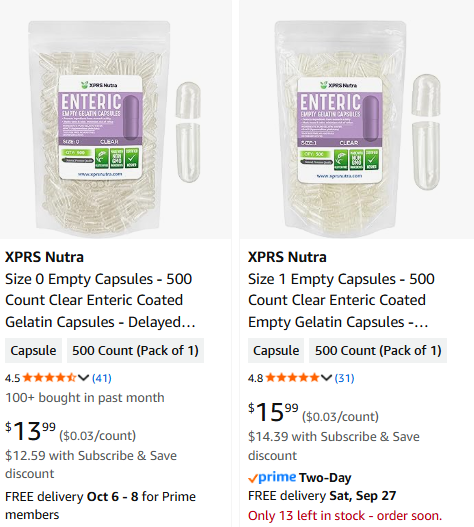
If you must have vegan, then you can get them at
Though they are currently sold out.
2 Likes
I think we have dealt with the basic errors in that post before.
1 Like
RapAdmin
#563
@John_Hemming has gotten me researching melatonin and mitochondria more. John, would you agree generally with the GPT5 summary below?
There was a review article that came out last year that was interesting:
and so I asked GPT5:
What does the broader related research on melatonin use with regard to health of mitochondria suggest in terms of potential benefits and doses of melatonin in human populations?
Response:
Here’s what the human literature (beyond that one Frontiers review) suggests about melatonin, mitochondria‐linked benefits, and dose ranges—plus where evidence is thin.
What benefits have actually been seen in people?
-
Direct mitochondrial readouts improved (small RCT, PD patients):
50 mg nightly for 3 months increased mitochondrial Complex I activity and respiratory control ratio and improved antioxidant enzymes vs. placebo. These are bona-fide mitochondrial endpoints (platelet/biochemical measures). (PubMed )
-
Exercise / skeletal muscle milieu (mainly mechanistic, some human data):
Trials around strenuous exercise report better PGC-1α response, higher glycogen repletion, and lower intramuscular triglyceride shortly after exhaustive exercise. Human work is supportive mechanistically, but dose timing is acute and studies are small; much of the detailed dosing comes from animal models. (Frontiers)
-
Systemic oxidative stress / inflammation (meta-analyses):
RCT meta-analyses in clinical populations (e.g., diabetes, metabolic disorders) show reductions in CRP/IL-6 and improved total antioxidant capacity; mitochondrial markers are seldom measured, but redox benefits align with mitochondrial protection. Typical doses in these trials are 3–10 mg/day, sometimes higher. (PubMed )
What dose ranges show signal (from human studies)?
-
0.3–3 mg qHS (physiologic/chronobiotic range): robust for sleep; mitochondria-specific endpoints rarely measured at these doses. PK is highly variable (bioavailability ~1–74%). (NCBI)
-
3–10 mg/day (common clinical trial range): frequently used in oxidative-stress/inflammation RCTs with biochemical antioxidant benefits, but again few direct mitochondrial assays. (PubMed )
-
10–20 mg/day: used in some adjunct trials (neuro/critical-care contexts); mixed endpoints, limited mitochondrial assays. (Contextualized by broader clinical reviews.) (PMC)
-
50 mg/day: the clearest human signal on mitochondrial function per se (↑Complex I, ↑RCR after 3 months). This is pharmacologic and above sleep doses. (PubMed )
-
>50–100 mg (short-term): seen in sports/acute protocols and some experimental settings; safety data exist for “higher-dose” melatonin in RCTs, but mitochondrial endpoints are sparse. (MDPI)
Practical takeaways (evidence-based, not medical advice)
- If your goal is mitochondrial adaptation (not just sleep), the most direct human evidence to date used 50 mg nightly for 3 months in PD patients and improved mitochondrial respiratory metrics. Generalizability to healthy adults or other diseases is uncertain, but this sets a proof-of-principle dose where mitochondrial readouts moved. (PubMed )
- For broader redox/anti-inflammatory support that plausibly benefits mitochondria indirectly, 3–10 mg/night has RCT-level signals in various populations—just note the lack of head-to-head mitochondrial assays at these doses. (PubMed )
-
Timing likely matters: mechanistic and exercise studies imply synergy with stressors/training and evening dosing for chronobiology, but avoid close proximity to meals (see cautions). (Frontiers)
Key cautions that affect “optimal” dosing
-
Glucose control: acute melatonin near meals impairs glucose tolerance, especially in MTNR1B risk-allele carriers and with late dinners—separate dosing from food (e.g., 2–3 h after last calories, at bedtime). (Oxford Academic)
-
Drug interactions: case-series data suggest possible INR elevation with warfarin; if combined, monitor INR closely. (PMC)
-
Pharmacokinetics vary hugely by formulation and person (bioavailability 1–74%), so effective tissue exposure at a given oral dose is uncertain. (NCBI)
-
Safety: higher-dose melatonin has been well-tolerated in many trials, but dosing far above sleep ranges should be medically supervised and tailored to context. (Wiley Online Library)
Bottom line
- There is no universally accepted “optimal” human dose for mitochondrial health.
- The strongest human mitochondrial signal so far used 50 mg/night for 3 months (PD cohort). For more general redox/anti-inflammatory aims that likely support mitochondria, 3–10 mg/night is where most RCTs sit—though they rarely assay mitochondrial function directly. Translate cautiously and consider genetics, meal timing, and interactions. (PubMed )
And related queries: https://chatgpt.com/share/68daf3fc-a848-8008-8c1e-e37f95648027
2 Likes
A shorter summary. I like short summaries. If I am interested in the short summary, I will read the entire article.
Key Findings
-
Mitochondrial Protection: Melatonin has been shown to protect mitochondrial membranes by preventing the opening of the mitochondrial permeability transition pore (mPTP), a process that can lead to cell death. This action supports mitochondrial quality control and energy production, which are vital for cellular function and longevity.
-
Antioxidant and Anti-Inflammatory Effects: As a potent antioxidant, melatonin scavenges free radicals and reduces oxidative stress, which is a significant contributor to aging and various diseases. Additionally, it modulates inflammatory pathways, potentially mitigating age-related chronic inflammation.
-
Cellular Maintenance and Longevity: The review discusses how melatonin influences cellular processes such as autophagy, apoptosis, and DNA repair. By enhancing these mechanisms, melatonin may help maintain cellular integrity and function over time, potentially extending lifespan.
Implications for Longevity
The article suggests that melatonin’s role in mitochondrial health and cellular maintenance positions it as a potential therapeutic agent for promoting longevity. While the evidence is compelling, the authors call for more clinical studies to confirm these effects in humans and to determine optimal dosing strategies.
In summary, melatonin’s multifaceted actions—ranging from mitochondrial protection to antioxidant and anti-inflammatory effects—underscore its potential as a modulator of aging processes. Further research is necessary to fully elucidate its role in longevity and to translate these findings into clinical applications.
2 Likes
I don’t think anything is wrong, but my personal focus is on mtDNA
1 Like
RapAdmin
#566
Digging further into this…
Prompt:
are there any easy ways for people to measure benefits from taking higher doses of melatonin, such as via inflamatory markers in blood tests?
GPT5 Response:
Good question — it’s very parallel to the rapamycin monitoring issue you asked about earlier. With melatonin, especially at higher-than-sleep doses (≥10–50 mg/day), people want to know if it’s “doing anything” for mitochondria, inflammation, or systemic stress.
Here’s a structured way to think about what can realistically be tracked in humans:
1. Basic, accessible inflammatory markers
These are widely available on routine blood panels and can show systemic redox/inflammatory status:
-
hs-CRP (high-sensitivity C-reactive protein)
– Melatonin has been shown in multiple RCTs/meta-analyses to lower CRP, especially in metabolic disease and sepsis contexts.
-
IL-6 and TNF-α
– Cytokines that melatonin often reduces in human and animal trials. IL-6 in particular is elevated with aging and chronic inflammation.
-
Neutrophil-to-lymphocyte ratio (NLR)
– A simple ratio from a CBC; melatonin has lowered NLR in critical illness studies, reflecting an anti-inflammatory tilt.
2. Oxidative stress & antioxidant status
Melatonin is both a direct ROS scavenger and an indirect antioxidant via SOD/GPx/CAT upregulation. Some labs offer:
- Total antioxidant capacity (TAC)
-
Malondialdehyde (MDA) or F2-isoprostanes (lipid peroxidation markers).
-
Glutathione (GSH/GSSG ratio) – an excellent redox balance marker, but usually requires specialty labs.
3. Mitochondrial function proxies
Not routine, but possible in research or specialty testing:
-
Lactate/pyruvate ratio – shifts may reflect mitochondrial oxidative metabolism efficiency.
-
ATP production or mitochondrial membrane potential in PBMCs (seen in Parkinson’s 50 mg melatonin RCT). This is research-grade, not a standard clinical test.
-
Complex I/IV activity assays (specialty labs, not routine).
4. Clinical-function readouts
Beyond blood, you can also monitor:
-
Sleep quality (actigraphy, wearables) – although at high doses melatonin may sedate more than optimize circadian rhythm.
-
Immune response (e.g., antibody titers after flu/COVID vaccines) – less studied with melatonin than with rapamycin, but plausible given its immune-regulatory role.
-
Blood pressure, HRV – some high-dose studies noted improved vascular tone and autonomic balance.
3 Likes
adssx
#567
Rethinking Melatonin Dosing: Safety and Efficacy at Higher-than-Usual Levels in Aged Patients with Sleep Disturbances and Comorbidities 2025
Background. Although melatonin is widely used in Sleep Medicine for its chronobiological action, its potent antioxidant and mitochondrial regulatory effects, as well as its immunomodulatory and anti-inflammatory functions, make it of interest as a cytoprotective agent in several chronic pathologies. These actions are evident at doses higher than those used for sleep disorders. Even at high doses, melatonin’s adverse effects are few, mild, and self-limited or resolve quickly after discontinuation of treatment. Based on its safety profile, we treated melatonin for sleep disorders in the presence of comorbidities with doses ≥ 40 mg daily.
Methods. This was a retrospective mixed observational analytical design comprising a retrospective uncontrolled cohort analysis and a cross-sectional study. Eighty-one patients (57 female) with sleep disorders ranging in age from 55 to 98 years (mean 74.4 years) were treated with melatonin 40 to 200 mg daily (mean 72.7 mg) were examined. Fifty-six percent of patients received treatment for more than 4 years. The control group for the cross-sectional analysis included 81 patients over 52 years of age, matched by age and sex and not receiving melatonin but having sleep disorders within the same period.
Results. A significant decrease was observed in arterial hypertension, ischemic heart disease and diabetes mellitus after melatonin administration. Analysis of clinical laboratory variables indicated no changes in the treated group versus the untreated group, except for a lower alkaline phosphatase concentration in patients who received melatonin.
Conclusions. These findings suggest a beneficial effect of cytoprotective doses of melatonin on the cardiovascular and metabolic profile in an aged population.
This study demonstrates that long-term administration of high-dose melatonin (40–200 mg/day) in older adults with sleep disorders is well tolerated and suggests that it is associated with improvements in arterial hypertension, ischemic heart disease, and diabetes mellitus, alongside favorable modulation of alkaline phosphatase levels without other laboratory alterations. Long-term clinical studies evaluating the safety of high-dose melatonin in elderly patients remain scarce. While our study provides long-term data supporting the safety of high-dose melatonin in elderly patients, further studies are needed to fully establish its long-term risks. The observed improvement in the studied conditions should be interpreted as correlations rather than proof of a protective effect, although they are consistent with the cytoprotective potential of supraphysiological melatonin dosing for improving cardiovascular and metabolic health in the elderly. Future prospective, long-term, randomized trials are warranted to confirm safety and efficacy as well as to define optimal dosing strategies.
3 Likes
adssx
#568
Melatonin suppresses cancer cell proliferation, DNA repair and expression of the oncogene TRIP13 2025
Here, we demonstrate that melatonin (MT), a hormone synthesized in the pineal gland, downregulates the expression of TRIP13, particularly in lung cancer cells with high expression of TRIP13. Moreover, this downregulation of TRIP13 by MT further inhibits the DNA repair proteins RAD51 and XRCC5, thereby impairing DNA repair via homologous recombination and non-homologous end joining.
That’s the opposite of what we want @John_Hemming? (although in cancer cells inhibiting DNA repair is good = makes them more vulnerable to radiation and cell death)
ChatGPT says that there might be a duality: selective “DNA repair suppression” as “Cancer cells often overexpress MTNR1B and oncogenic pathways (e.g., TRIP13, PI3K/AKT, NF-κB) that melatonin suppresses.” while “Healthy cells rely more on antioxidant and circadian signaling, where melatonin acts as a protector.”
These things can be quite complex. If less DNA damage is done then the proteins that are part of DNA repair are less likely to be expressed.