Timing of Tirz or Reta and Rapamycin: If you’re taking Rapa and shots, do you find the timing matters?
I recently started Tirz at 1 mg on Saturdays, but when I then took my usual biweekly Rapamycin I felt pretty rough. Could be coincidence? Was thinking of pushing my Rapa does to Wednesday to avoid this.
Zxc
#122
How do you take a glp1 when rapamicyn raises glucose levels ?
LukeMV
#123
Im stopping Ivabradine to see if my cardio fitness feels any better.
Thats great your RHR came down. Must be doing something right.
1 Like
LukeMV
#124
Honestly the thought of them interacting never once crossed my mind. I really doubt it matters or what you describe is related. It’s certainly never been studied.
1 Like
Yes it might. I think taking rapamycin a few days before GLP1 agonist is a good strategy. GLP1 agonists surprisingly activate mTOR in certain ways, but it is beneficial the way it does it.
They might additively combine well, or they might partially cancel each other out. Until we know for sure I personally do not use them on the same day.
Read a summary of a study I did on GLP1s and longevity here: Study behind paywall, can anyone find the full study? "Unlocking longevity with GLP-1: A key to turn back the clock?" - #10 by AustraliaLongevity
1 Like
I assume you mean ‘why’ not ‘how do you take’ because I take it as an injection. only 5% of people have glucose issues as a side effect of Rapamycin Side Effects of Rapamycin (part 2)
If one does, surely that would be an excellent reason to take a GLP1 RA with Rapa, in addition to all the other health benefits of GLP1.
In fact my glucose was disregulated BEFORE I started taking Rapa and has continued to be. Tirzepatide has brought it down significantly.
2 Likes
Now I’m feeling the ideal level of appetite loss and more energetic but I still have been sleeping more than ideal.
Retatrutide has way better side effects than semaglutide which is way more important if you don’t intend to take these things weekly. I’ve had vomiting on semaglutide (and the appetite loss from semaglutide disappears in 2-3 days).
It’s nice to not think about food so much and to only need one real meal a day and also only to be able to eat soft foods. I feel dumb for not doing GLP-1’s more last year due to side effects, but [given some of my sub-ideal blood panels - btw canagliflozin does not seem to have helped, but I try anyways] I know I have to do them more.
None of the fatigue from the first time (I injected too much the first time, was underwhelmed the second time, the 3rd time was only a bit over 2mg)
even posted this in a signal thread and there looks like there are A LOT more people in that post-rat community who are doing retatrutide, off-hand
===
July 28: a conference day! and I still feel so full that I could only eat small handfuls of conference food (like, just one hummus sandwich). and then some harissa sauce at the end. I love my reduced appetite so much.
5 days since injection and still feeling full! semaglutide never gave me this privilege
==
July 29: 6 days since injection, appetite gone for first half of day, but I could ease into almonds for once, and then could eat a little bit more salad/avocado/chipotle sauce at pillar.vc event than I could on previous days, tho beans are still hard
[i think 6 days in after 2.5mg injection is what i’d like to feel like ALL the time]
Wow, but when your appetite returns after the 6.5-ith day… IT REALLY RETURNS…
Try half that dose, twice a week.
1 Like
Ok
But each injection is an exposure route fpr MPs
Appetite mostly gone today
My lymphocyte count is 1.1, unusually low. Neutrophils higher
Still haven’t lost weight, maybe half a pound
Oh I had low RBC TOO
Short answer
Neutrophils bounce back first. Within ~24 h of re-feeding, circulating neutrophil numbers and most functional read-outs (chemotaxis, oxidative burst) are already at—or very close to—pre-fast levels, whereas lymphocyte counts do not fully normalize until several days later. So after one day of eating again following a week-long fast you can expect your neutrophil compartment to look almost “recovered,” while your lymphocyte compartment will still be climbing back toward baseline.
Why neutrophils recover faster
| Feature |
Neutrophils |
Lymphocytes |
| Normal half-life in blood |
6–18 h |
Days → years depending on subset |
| Bone-marrow reserve |
Large pools of mature cells ready to demarginate |
Far smaller reserve of ready-to-circulate cells |
| Signals triggered by re-feeding |
Insulin, IGF-1, mTOR and above all G-CSF rapidly mobilize marrow neutrophils |
IL-7/IL-15 driven homeostatic proliferation plus chemokine-directed “re-export” from bone marrow and lymphoid tissues—slower processes |
| Human data after short fast |
Chemotactic index, H₂O₂ production and numbers back to baseline within 4 h of re-feeding in young adults |
Lymphocyte subsets did rise with feeding but were still significantly below pre-fast values over the same interval |
| Animal data after longer fast / CR |
Neutrophil counts surge within 24 h of re-feeding (demargination + production) |
Global lymphopenia reverses inside the first week, not the first day |
Physiology behind the time-lag
-
Neutrophils
- A one-week fast diminishes circulating neutrophils chiefly by pulling them back into marrow and by slowing granulopoiesis.
- Re-feeding causes a spike in glucose, insulin and IGF-1, turning mTOR back on and raising G-CSF. Those signals trigger (i) demargination of the sequestered pool and (ii) release of band and segmented neutrophils that were already mature in the marrow. Because their maturation pipeline is only ~5 days long, many “near-mature” cells are immediately available.
-
Lymphocytes
- During fasting, T- and B-cells redistribute out of blood to bone marrow and secondary lymphoid tissue to save energy. Proliferation stalls because IL-7/IL-15 and mTOR activity fall.
- When calories return, chemokine gradients reverse, but re-export plus renewed homeostatic proliferation take several cell cycles (2–4 d minimum). Mouse work shows full repopulation of blood, spleen and lymph nodes by about day 3–7 of ad-lib feeding after a prolonged calorie deficit.
Practical implications
- A single day of normal eating will nearly restore innate antibacterial defenses (neutrophils), but adaptive immunity (T- and B-cell–mediated responses, antibody production) may still be transiently below par.
- The kinetics are slower in older adults: in the Walrand study neutrophil chemotaxis rebounded in young adults after 4 h, but remained depressed in the elderly after the same meal .
- Big swings in calories/weight can transiently skew the neutrophil-to-lymphocyte ratio (NLR). If you draw labs right after re-feeding, expect a higher NLR than your long-term baseline.
Caveats
- Human data for exactly 7-day water fasts followed by 24 h of re-feeding are scarce; most controlled studies use 36–72 h fasts. The direction and relative speed, however, are consistent across fasting lengths.
- Micronutrient status (iron, B-vitamins), hydration, and the macronutrient composition of the re-feed can all modulate hematopoietic recovery. Severe protein restriction, for example, can blunt lymphocyte rebound even when calories return.
- If the fast was extreme enough to deplete marrow stores (rare in one week), neutrophil recovery could be a little slower (36–48 h).
Bottom line: Your neutrophil army is back on patrol within hours of that first re-feed day; your lymphocyte corps needs several more days to regroup.
What normally happens to RDW after a brief re-feed
| Time-point |
Dominant processes in the red-cell compartment |
Expected direction of RDW (CV or SD) |
| End of a 7-day fast |
• Erythropoiesis suppressed (low EPO/mTOR) |
|
| • No reticulocyte influx → older, slightly smaller cells dominate |
Mild rise (≈ +0.5–1 percentage point) because the size-spectrum narrows at the upper end while the smallest, “aged-out” cells accumulate |
|
| +24 h of eating again |
• Plasma volume expands; light meal itself slightly narrows the histogram |
|
| • Marrow has not yet released the first reticulocyte “wave” (takes ≥ 48 h) |
Largely unchanged or even ↓ ~0–0.3 pp (hemodilution effect seen after a meal in healthy volunteers) |
|
| Day 2-5 of re-feeding |
• Reticulocytes (macrocytic) begin to spill out; anisocytosis peaks because young large cells mix with the older, smaller fast-era cohort • RDW tracks the retic count |
Transient spike (often reaching high-normal or mildly elevated range, e.g. 14 → 15 %) |
| Week 2-4 |
• Fast-era cells (120-d lifespan) are increasingly replaced by post-refeed normocytic cells |
Gradual normalization as the size distribution re-homogenizes |
Why the first 24 h show little change
-
Reticulocyte kinetics: Even in vigorously stimulated marrow, it takes ≈ 2–3 days before newly made reticulocytes appear in peripheral blood; peak regenerative response is 6–10 days.
-
Meal-related dilution: A single mixed meal expands plasma volume and narrows red-cell histograms slightly; RDW fell by ~0.4 % within one hour in a controlled study of healthy adults.
-
Magnitude of the prior anemia: A 7-day fast rarely depletes iron or folate stores enough to create pronounced micro- or macro-cytosis, so the pre-existing anisocytosis is modest to begin with.
Taken together, the net effect at +24 h is either:
-
No measurable change (most common); or
-
A tiny fall—often below the analytical variation flag of automated counters—due to plasma-volume expansion slightly outweighing early regenerative changes.
Practical interpretation
-
Do not expect RDW to “bounce” like neutrophils do: The index reflects the mix of cell ages and sizes, and those pools turn over on the scale of weeks, not hours.
-
A higher RDW two–three days later is normal: It signals the anticipated reticulocyte surge rather than a new problem.
-
Serial CBCs: If you are monitoring recovery, draw the next panel at ≥ 48 h. Earlier sampling adds little insight but can be misleading.
Bottom line: One day of re-feeding after a week-long fast that produced mild anemia is too soon for erythropoiesis to reshape the size spectrum of circulating red cells; RDW therefore stays roughly where it was—or dips slightly—before climbing during days 2-5 as reticulocytes enter the bloodstream.
8 days later and appetite still very low, I can probably have several more days before redosing
no weight loss
===
15 days later and my appetite is still lower than it was most of the time last year so I still don’t need any re-injections!
The brain is the master regulator of food intake and energy balance.
Brain control of energy homeostasis: Implications for anti-obesity pharmacotherapy
Emerging pharmacotherapies that mimic gut peptides, such as glucagon-like peptide-1 (GLP-1), have shown that targeting specific neurons in the brainstem and hypothalamus is an effective strategy for inducing weight loss and lowering the risk of obesity-associated comorbidities
https://www.cell.com/cell/fulltext/S0092-8674(25)00677-4
6 Likes
Wow that’s quite the wrap up of ALL the pathways. Much of it is beyond me but just the range of interactions and new knowledge is interesting. I loved this line “… it seems highly unlikely that the obesity epidemic can be explained by a widespread drop in willpower happening in the late 1970s.”
2 Likes
This is about Tirzepatide a dual agonist. I’d consider that these mechanistic properties are also in Retatatrutide a triple agonist (+ the benefit of GCGR)
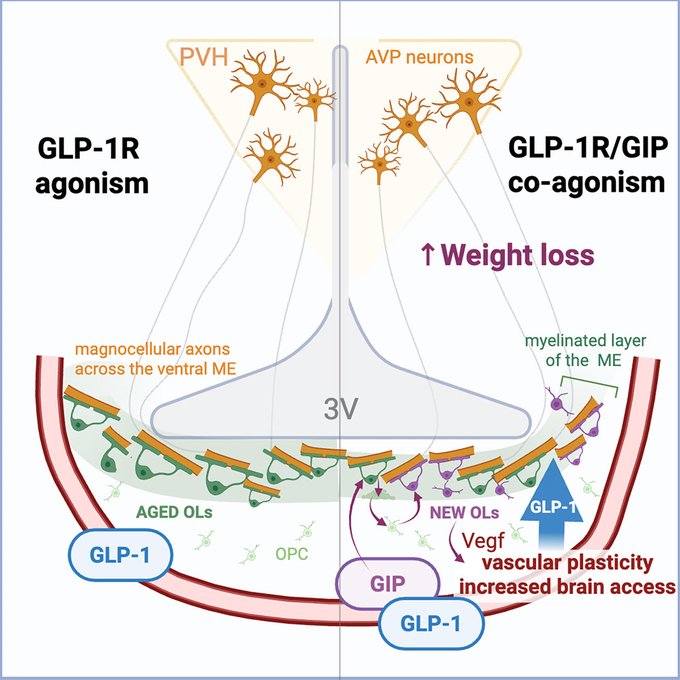
Glucose-dependent insulinotropic polypeptide receptor signaling in oligodendrocytes increases the weight-loss action of GLP-1R agonism
Highlights
• GIPR in oligodendrocytes (OLs) regulates median eminence (ME) oligodendrogenesis
• GIPR in OLs is required for full weight-loss efficacy of GIPR/GLP-1R co-agonism
• GIP increases brain access of GLP-1R agonists to vasopressin axons in the ME
• PVH vasopressin neurons are required for GLP-1R-agonist-induced weight loss
Summary
The next generation of obesity medicines harness the activity of the glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptors (GIPR and GLP-1R), but their mechanism of action remains unclear. Here, we report that the GIPR is enriched in oligodendrocytes and GIPR signaling bidirectionally regulates oligodendrogenesis. In mice with adult-onset deletion of GIPR in oligodendrocytes, GIPR agonism fails to enhance the weight-loss effects of GLP-1R agonism. Mechanistically, GIPR agonism increases brain access of GLP-1R agonists, and GIPR signaling in oligodendrocytes is required for this effect. In addition, we show that vasopressin neurons of the paraventricular hypothalamus are necessary for the weight-loss response to GLP-1R activation, targeted by peripherally administered GLP-1R agonists via their axonal compartment, and this access is increased by activation of the GIPR in oligodendrocytes. Collectively, our findings identify a novel mechanism by which incretin therapies may function to promote synergistic weight loss in the management of excess adiposity.
https://www.cell.com/cell-metabolism/fulltext/S1550-4131(25)00355-9
3 Likes
Cool stuff, we’re getting better at designing those classes of drugs. Basically, there’s no excuse to not agonize GIP if you hit GLP1. I’m personally curious to see what a combination of agonizing GLP1, GIP and amylin would do.
1 Like
Do you take Nebivolol? I thought I saw you took a beta blocker in another thread. If you do, what are your thoughts? Any negative affects on training?
I got some Cagrilintide in last week. Have taken a 1.0mg dose on Sunday along with my usual 2.0mg Reta shot.
Looks like it has some interesting benefits.
The Role of the Amylin Analogue Cagrilintide in Bone Metabolism (RAMBO)
https://clinicaltrials.gov/study/NCT07010432
1 Like
Davin8r
#140
The problem with cagrilintide is that for so many, it just flattens them with fatigue and sleepiness. I just see it over and over again in the forums and experienced it myself even at a small fraction of the usual starting dose. There are obviously plenty who can tolerate it, and they’re the lucky ones. Either the pharma studies severely underestimate fatigue as a side effect, or there is something different about the UGL versions that make this side effect more common.
2 Likes