KarlT
#89
The Effect of Semaglutide on Mortality and COVID-19–Related Deaths: An Analysis From the SELECT Trial
https://www.jacc.org/doi/10.1016/j.jacc.2024.08.007
These findings highlight the mortality benefit of semaglutide across a broad population of patients with CV disease and obesity. The robust reduction in non-CV death, particularly infectious deaths, was surprising and perhaps only detectable because of the COVID-19–related surge in non-CV deaths; however, these findings reinforce that overweight and obesity increase the risk of death because of many etiologies, which can be modified with potent incretin-based therapies like semaglutide.
3 Likes
A_User
#90
What was the effect? I don’t want to click.
1 Like
I would imagine by the price, packaging and top color (usually means nothing) they are from QSC. Here is your initial test report for that batch. It tested out at 99.389% and 11.43 mg that was validated by customer independent testing to be dose correct, but the purity degraded to ~98% due to humidity control issues. So if you were splitting the vile you are taking closer to 6 mg every 5 days which is essentially at max dose. Listen to this group. Titrate slowly and listen to your body. You are compounding so you are not stuck with having to make 2.5 mg jumps that can make you sick.
https://janoshik.com/tests/45822-Tirzepatide_10_mg_42IUSJYPJ3L6
3 Likes
Currently doing 0.5mg a day.
I still feel tired. My HRV has fallen from 80 to 25 and my RHR is higher. I started nebivolol 5mg to fell better.
I started vortioxetine because for few weeks I fell pretty bad mentally (used to be on antidepressants for years, was on nothing for about 2 months), so my HRV is not going to increase soon.
adssx
#94
The effect of GLP-1RAs on mood is weird; some people report feeling depressed, others the opposite.
Many recent papers looked at suicide ideation:
-
Exploration of the potential association between GLP-1 receptor agonists and suicidal or self-injurious behaviors: a pharmacovigilance study based on the FDA Adverse Event Reporting System database 2024 “In analysis of TTO based on parameter distributions and the valid cases, the median TTOs (Interquartile range) for SSIBs associated with semaglutide, liraglutide, dulaglutide, and exenatide were 15.5 (0.5–24.25, n = 18), 9.5 (0.5–61.5, n = 19), 35.5 (6.5–60.5, n = 11), and 2.5 (1.5–169.5, n = 4) days, respectively (albiglutide was not included in the TTO analysis due to insufficient data).”
- I don’t know how to interpret that.
-
Real-World Data in Pharmacovigilance Database Provides a New Perspective for 2 Understanding the Psychiatric Adverse Events Associated with Glucagon-Like Peptide-1 3 Receptor Agonists (GLP-1 RAs) Exposure 2024: “Positive signals of suicidal ideation 130 [ROR=1.38 (1.14-1.66)] and depression/suicidal [ROR=3.96 (2.29-6.82)] was observed for 131 semaglutide.”
- Based on Table 3, I understand that for suicidal ideation: semaglutide > liraglutide > exenatide > tirzepatide > dulaglutide, while for depression/suicidal: semaglutide > liraglutide > exenatide > dulaglutide.
-
Risk of Suicide, Hair Loss, and Aspiration with GLP1-Receptor Agonists and Other Diabetic Agents: A Real-World Pharmacovigilance Study 2024: “Semaglutide [ROR = 0.60 (0.51–0.71)] and liraglutide [ROR = 0.28 (0.23–0.35)] had higher suicidal events than DPP4is and SGLT2is.”
-
Exploring the association between suicidal thoughts, self-injury, and GLP-1 receptor agonists in weight loss treatments: Insights from pharmacovigilance measures and unmasking analysis 2024: “After unmasking, 271 cases where individual GLP-1 RA were implicated showing liraglutide (n = 90; Reported Odds Ratio (ROR) = 1.64), exenatide (n = 67; ROR = 0.80), semaglutide (n = 61; ROR = 2.03), dulaglutide (n = 45; ROR = 0.84), tirzepatide (n = 5; ROR = 1.76) and albiglutide (n = 2; ROR = 0.04).”
- So semaglutide > tirzepatide > liraglutide > dulaglutide > exenatide > albiglutide?
-
Glucagon-like Peptide-1 Receptor Agonists and Suicidal Ideation: Analysis of Real-Word Data Collected in the European Pharmacovigilance Database 2024: “Disproportionality analysis found a higher reporting probability of suicidal events for semaglutide than dulaglutide (ROR, 2.05; 95%CI, 1.40–3.01) and exenatide (ROR, 1.81; 95%CI, 1.08–3.05). In the same way, liraglutide was associated with a higher reporting probability of suicidal events than dulaglutide (ROR, 3.98; 95%CI, 2.73–5.82) and exenatide (ROR, 3.52; 95%CI, 2.10–5.92). On the contrary, a lower reporting probability was found for semaglutide than liraglutide (ROR, 0.51; 95%CI, 0.38–0.69).”
- So semaglutide > liraglutide > exenatide > dulaglutide?
So the above papers seem to conclude that in terms of suicidal ideation risk, from the highest to the lowest risk we have: semaglutide > liraglutide > exenatide > dulaglutide > albiglutide.
Weirdly that’s the opposite of the BBB-crossing properties (transport into whole brain within one hour), per Brain uptake pharmacokinetics of albiglutide, dulaglutide, tirzepatide, and DA5-CH in the search for new treatments of Alzheimer’s and Parkinson’s diseases 2023:
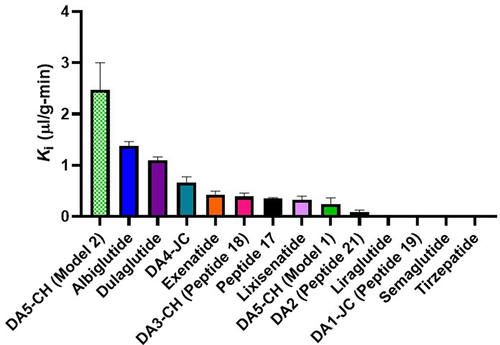
So the more the drug crosses the BBB the lower the suicidal ideation risk?
However, in terms of weight loss, I think it’s also semaglutide > liraglutide > exenatide > dulaglutide > albiglutide. So I thought that maybe the higher suicidal ideation risk in semaglutide users might be due to semaglutide users being more obese or overweight and obesity being associated with a higher risk of suicidal ideation. But apparently, that’s not the case (it could even be the opposite!): “Obesity is not associated with suicide ideation among U.S. young men and women. Overweight is associated with a lower risk of suicide ideation among young men.” The relationship between obesity and suicide ideation among young adults in the United States 2022. So it’s probably not that.
Still, the above order is also the one for HbA1c lowering potency. So, semaglutide users might have more advanced diabetes than exenatide users. Apparently “people with diabetes have double the risk of suicide or intentional self-injury compared with the general population” (not a great source). So this might explain the differences between GLP-1RAs  .
.
So it’s very unclear… In the past two months, in JAMA only, different articles and editorials had opposite conclusions:
-
Glucagon-Like Peptide-1 Receptor Agonists and Suicidality—Two Important Pieces of Data but an Incomplete Puzzle:
-
GLP-1 Receptor Agonist Use and Risk of Suicide Death: “This study provides reassuring data showing that those initiating GLP-1 receptor agonist use were not at increased risk of suicide death, although the study could not assess small risk increases.”
-
Psychiatric Safety of Semaglutide for Weight Management in People Without Known Major Psychopathology: “The results of this post hoc analysis suggest that the risk of developing symptoms of depression or suicidal ideation/behavior was similar between semaglutide, 2.4 mg, and placebo.”
- GLP-1 RA Drugs Not Associated With Increased Suicidal Thoughts
-
GLP-1 Receptor Agonists and Suicidality—Caution Is Needed: “Waiting for more precise data, GPL-1 receptor agonists, and appetite suppressants in general, should be prescribed with great caution in patients with a history of depression or suicidal attempts, while in patients with new onset of depression without other apparent precipitants, immediate discontinuation of GLP-1 receptor agonists should be considered.”
-
Disproportionality Analysis From World Health Organization Data on Semaglutide, Liraglutide, and Suicidality: “Treatment with semaglutide or liraglutide regardless of indication or treatment duration.”
- GLP-1 RA Drugs Not Associated With Increased Suicidal Thoughts
My two cents: There are intraclass differences, and grouping all GLP-1RAs into one category might lead to incorrect conclusions. People with a history of depression might prefer dulaglutide to semaglutide for glycemic control. For weight loss: unclear.
@DrFraser: what do you think?
5 Likes
A_User
#95
I don’t know if you’ve seen or linked this one, published in Nature:
In patients with overweight or obesity (mean age 50.1 years, 72.6% female), semaglutide compared with non-GLP1R agonist anti-obesity medications was associated with lower risk for incident (HR = 0.27, 95% CI = 0.200.32–0.600.36) and recurrent (HR = 0.44, 95% CI = 0.32–0.60) suicidal ideation, consistent across sex, age and ethnicity stratification. Similar findings were replicated in patients with T2DM (mean age 57.5 years, 49.2% female). Our findings do not support higher risks of suicidal ideation with semaglutide compared with non-GLP1R agonist anti-obesity or anti-diabetes medications.
adssx
#96
I saw it but chose not to mention it as it compared semaglutide to “non-GLP1R agonist anti-obesity medications”: bupropion, naltrexone, orlistat, topiramate, phentermine and setmelanotide. I don’t think these drugs are great for your body. So I’m not surprised that semaglutide does better compared to those. I also think they’re not as effective for weight-loss but more affordable, so people on them could be poorer and may be subject to more financial strain? (Financial strain is associated with a higher risk of suicide: Financial Strain and Suicide Attempts in a Nationally Representative Sample of US Adults | American Journal of Epidemiology | Oxford Academic )
So I think this study is irrelevant unfortunately.
The best would be to look at people with T2D but neither overweight nor obese and compare individual GLP1-RA to SGLT2.
1 Like
A_User
#97
They did control for socioeconomic determinants of health, and with an anti-diabetes medication comparison as well, including SGLT2i’s. That analysis wasn’t in obese people either although the ozempic group had more obesity (but propensity score matching controls for it?).
The semaglutide group compared to the non-GLP1R agonist anti-diabetes medication group (insulin, metformin, sulfonylureas, alpha glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, sodium/glucose co-transporter 2 (SGLT2) inhibitors)
adssx
#98
Ah you’re right so they did two studies: One on "240,618 patients with overweight or obesity who were prescribed semaglutide or non-GLP1R agonist anti-obesity medications and another one “in 1,589,855 patients with T2DM”.
I missed the adjustment for socioeconomic determinants of health. Thanks for correcting my mistake. Still, I think the study on obese/overweight people is not helpful for the reasons I mentioned previously.
The one with T2DM is more interesting. They compared semaglutide to non-GLP1R agonist anti-diabetes drugs: insulin, metformin, sulfonylureas, alpha glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, sodium/glucose co-transporter 2 (SGLT2) inhibitors.
Result: “The semaglutide group had a significantly lower risk for incident suicidal ideation than the matched non-GLP1R agonist anti-diabetes medication group (0.13% versus 0.36%; HR = 0.36, 95% CI = 0.25–0.53).”
However, they lumped all other antidiabetic drugs together. A very heterogenous category. According to Table 2 |, 43.6% of patients were on insulin and 61.5% on metformin. So is the real conclusion “Adding semaglutide to your T2DM regimen lowers the suicide risk compared to nonuse”?
I still think this paper isn’t helpful.
3 Likes
I suspect that patient characteristics and a proper control group lacking are big factors in the conclusions.
If you are on semaglutide, obesity is more likely involved than other drug selections. Although a difference between tirzepatide and semaglutide is interesting as the demographic on those two are likely much closer than with the older GLP-1’s.
Overall, I’m not at all convinced one way or the other. Will wait for more information before thinking this increases or decreases risks of suicide.
3 Likes
Am I wrong in thinking that all these are fairly tiny effects vs nonusers of any of these drugs? And are these effects, small as they are, more or less relevant based on why they are taken, for weght loss, for t2dm, for longevity? That is assuming these effects are real in the first place rather than statistical artifacts and noisy data.
adssx
#101
4x seems massive to me.
At the population level, maybe, but for people with a history of depression or suicidal attempts, definitely not. Better be alive and obese than thin but dead.
Of course, we don’t know yet if the effects are real.
Assuming these effects are real, and even if they might be small, it would be super interesting to know by which mechanism(s) drugs that do not cross the BBB have an impact on mental health. It might unlock other benefits (esp. re. neuroprotection).
3 Likes
Why do you say stuff like this?
4 times what?
What is the absolute risk?
adssx
#103
Some ROR are around 4x here: Experience with GLP-1s - #94 by adssx
Again, nothing is proven, these are association studies. But if true that would be massive.
1 Like
I am not good at interpreting the results.
What do you think is the probable actual risk based on these studies, 1 in 10,1 in 100, etc?
adssx
#105
It’s a relative risk compared to non-use. So IF this is confirmed and causal, then it means that using drug Z would increase your risk of suicidal ideation by 4x. So, if you’ve never thought about this in your life, it will probably not change anything for you. If you have suicidal ideation once per year, then you might have it once per quarter. (I’m oversimplifying.)
3 Likes
dicarlo2
#106
1 Like
adssx
#107
Is this action detrimental or not in non diabetic / non overweight individuals?
Interesting bit:
In anaesthetized pigs, neither cervical vagotomy, adrenergic blockers (alpha, beta, or combined alpha-beta blockade), ganglionic blockade (hexamethonium), nor inhibition of hyperpolarization-activated cyclic nucleotide–gated (HCN) channels (ivabradine) abolished the marked chronotropic effect of GLP-1.
3 Likes
Davin8r
#108
“It is tempting to speculate that treatment of GLP-1 RAs might be combined with drugs targeting calcium clock dynamics or intracellular calcium levels, such as calcium channel blockers, or other downstream effectors, to prevent heart rate increases in patients receiving GLP-1 RAs where this may be considered beneficial.”
So, diltiazem might be the best choice (perhaps at a low dose) to reverse the elevated heart rate without affecting weight loss.
1 Like